Microneedle Flu Vaccine Market Size & Share Report 2034
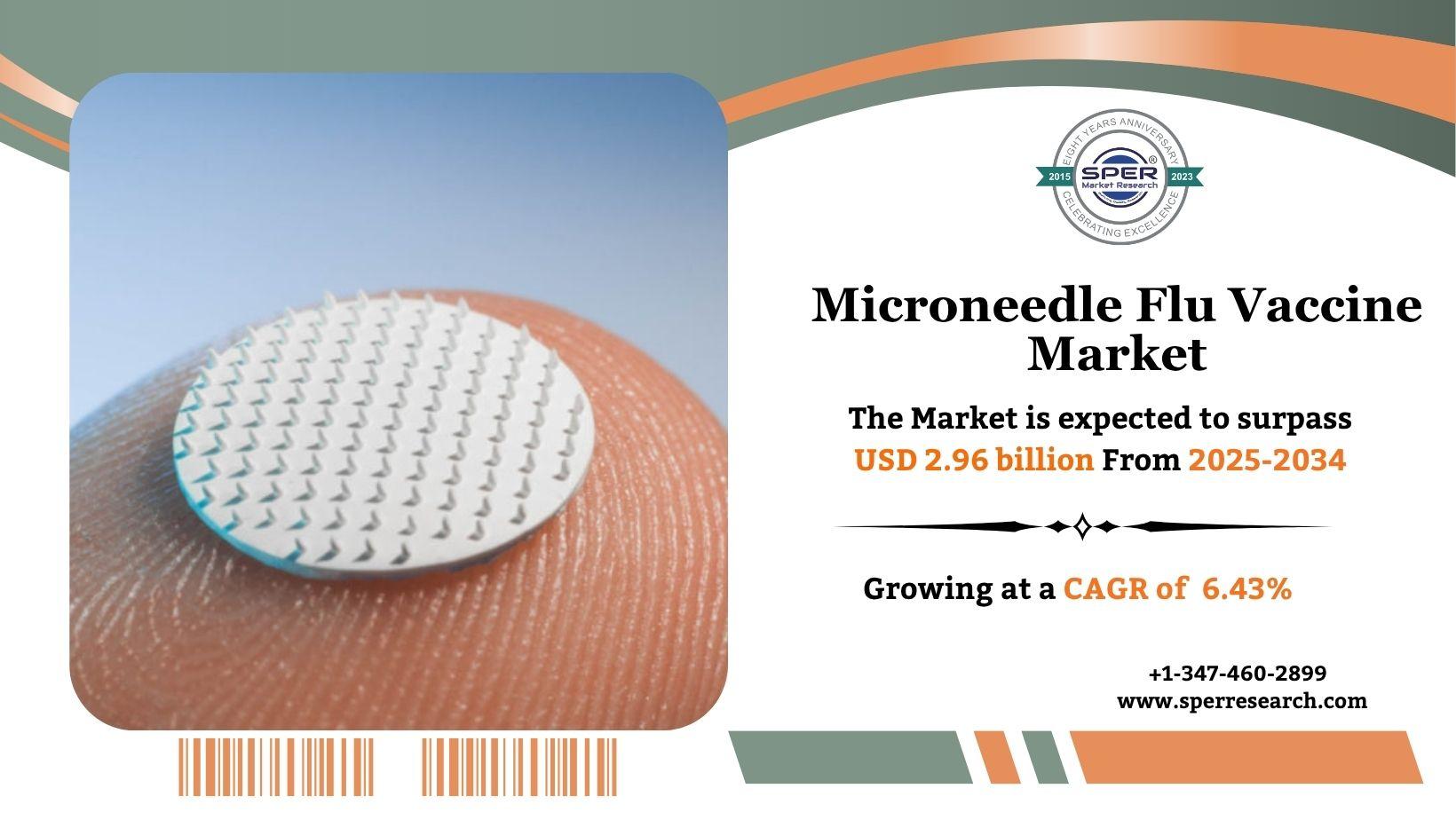
Microneedle flu vaccines deliver the influenza vaccine using tiny needles that penetrate only the outer layers of the skin. These microneedles are either coated with the vaccine or made to dissolve upon application, releasing the vaccine into the skin’s immune-rich areas. This method enhances immune response by targeting specialized cells in the epidermis and dermis. Unlike traditional injections, microneedle patches are nearly painless, easy to use, and may not require trained medical staff or cold storage. Clinical studies show they offer similar or even better protection, making them a promising and convenient alternative to conventional flu vaccination methods, with potential for self-administration.
According to SPER Market Research, ‘Microneedle Flu Vaccine Market Growth, Size, Trends Analysis – By Product Type, By Vaccine Type - Regional Outlook, Competitive Strategies and Segment Forecast to 2034’ the Global Microneedle Flu Vaccine Market is estimated to reach USD 2.96 billion by 2034 with a CAGR of 6.43%.
Drivers:
The growing global cases of seasonal influenza have led to an increased demand for more effective and user-friendly vaccine delivery systems. Microneedle flu vaccines offer a minimally invasive alternative to traditional injections, appealing to individuals who fear needles or prefer self-administration. Their design allows for easy use at home without the need for trained healthcare professionals, making mass vaccination efforts more accessible, especially in rural or underdeveloped areas. Furthermore, these patches are often more stable and less dependent on cold chain logistics, reducing transportation and storage costs. Strong support from public health agencies and rising investment in microneedle technology have accelerated advancements, further improving vaccine efficacy, patient compliance, and expanding potential applications across broader age groups.
For More detailed Analysis in PDF Format - https://www.sperresearch.com/report-store/microneedle-flu-vaccine-market.aspx?sample=1
Restraints:
While microneedle flu vaccines present a promising shift in vaccine delivery, several challenges continue to restrict widespread adoption. The relatively slow dissolution rate of some microneedles, which may require several minutes or more, could limit user convenience and acceptance. Additionally, there is a possibility of mild side effects such as skin irritation, redness, or scarring at the application site, which can deter repeat usage. Regulatory complexities also act as a barrier, since microneedle products often lie in a gray area between medical devices and pharmaceuticals, slowing down approval processes. Furthermore, the initial investment needed for manufacturing scale-up, packaging, and integration into existing immunization frameworks can be substantial, especially in lower-income countries with limited healthcare infrastructure.
North America leads the microneedle flu vaccine market, driven by its robust healthcare infrastructure, significant R&D investments, and high vaccine adoption rates. Some key players- Becton, CosMED Pharmaceuticals Co., Ltd, Debiotech S.A, Dickinson and Company, FluGen, Inc, MERCK & CO., INC, Microdermics Inc, NanoPass Technologies Limited, PFIZER, INC, TSRL Inc, Vaxess Technologies. and others.
For More Information, refer to below link: –
Microneedle Flu Vaccine Market Demand
Related Reports:
Postpartum Health Supplements Market Growth
Follow Us –
LinkedIn | Instagram | Facebook | Twitter
Contact Us:
Sara Lopes, Business Consultant — USA
SPER Market Research
+1–347–460–2899
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Παιχνίδια
- Gardening
- Health
- Κεντρική Σελίδα
- Literature
- Music
- Networking
- άλλο
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



