Protein accounts for 18-20% of the total mass of the human body and is the main carrier of human life activity. Protein has a range of complex functions in the organism, and many human diseases are closely related to the functional regulation of protein. For example, Parkinson’s disease (PD). It is understood that protein-stable imbalance disorders associated with mitochondria are important factors that lead to PD disease. Recent studies have shown that protein / sulfur dioxide heterogeneizes can reduce endoplasmic reticulum stress and regulate unfolded protein stress signal pathway changes, which can provide neuroprotective effects in cell PD models, effectively improving Parkinson’s syndrome epithetics. Cancer is another disease closely related to protein function. Studies have found that the occurrence of cancer is related to the overexpression/downregulation of specific enzymes in organisms. Therefore, using protein as a drug to directly treat diseases is a promising treatment method. However, the poor stability of the tertiary structure of protein drugs, easy to cause immune reactions, easy aggregation and inactivation in serum, and short biological half-life also limit its development. With the continuous innovation of modern nanotechnology, the rapid development of the technology for using nanoparticles as carriers to increase the load, targeting, release and degradation of drug molecules has brought new ways for clinical medicine to solve drug targeted release. Therefore, this article will provide an overview of the nano-carrier design strategy and mechanism for the delivery of intra-cellular protein drugs based on natural materials.
Natural Nanocarriers
Natural nanocarriers have attracted much attention in the field of drug delivery due to their variety, good biocompatibility, and acquisition.
Nanoparticles Based on Proteins
Protein-based nanoparticles have good biocompatibility, and their composition units are mainly amino acids, so protein-based nano-particles have multi-site functional properties, and can be precisely genetically modified to be used for targeted drug delivery. Through the method of self-assembly, multifunctional peptide components can be combined with therapeutic drugs to prepare functionalized protein nanocarriers.
Complex of Protein
Many proteins extracted from animal or plant bodies can be used as nanoparticles for the preparation of protein-based nanocarriers. For example, Bombyx mori silk protein and recombinant spider silk protein (eADF4) are considered to be low-toxicity and controllable protein drugs for cells, and lysosomes as model protein drugs are loaded on the surface of nanoparticles through electrostatic interaction, and the pH value and ionic strength The changes can realize the controlled release of drug molecules. Bovine serum albumin (BSA) is non-toxic and biodegradable, and can be used to prepare drug carriers. Based on this property, the researchers synthesized a BSA nanoparticle loaded with a drug protein (NELL-1), which was then coated with chitosan (CS) to enhance its stability. The encapsulation efficiency of the nanosystem is very high and the drug molecules can maintain sustained release within 8 days. Elastin peptides (ELPs) are produced by genetic recombination and can dissolve or self-assemble into aggregates near the transition temperature (Tt). ELPs can be assembled into nanoparticles through gene fusion and cell growth factor (KGF) at physiological temperature. Studies have found that this ELPs-KGFs nanoparticles can promote tissue re-epithelialization and have a good effect on improving wound healing in diabetic mice.
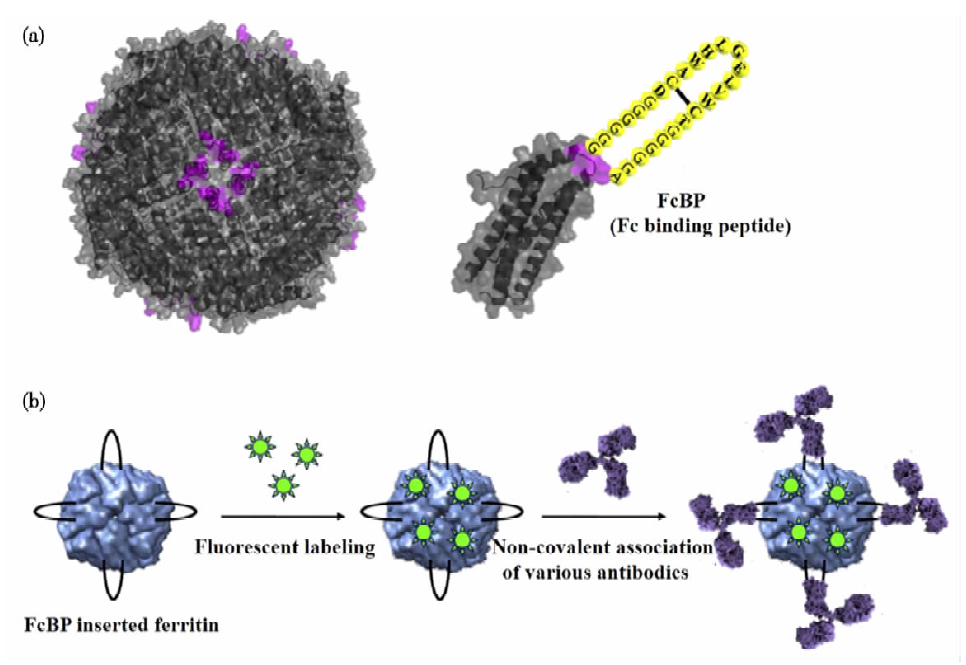
Protein Cage

Protein cages are attractive nanoscale drug delivery vehicles with a diameter of less than 30 nm, and are widely used for the delivery of protein drugs. Most protein drug molecules can be attached to the surface of protein cages by covalent interaction or gene fusion. For example, researchers introduced a fusion protein (Fc)-binding peptide (FcBP) into the loop of a self-assembling protein cage. In this cage, FcBP-containing ferritin binds human and rabbit immunoglobulins (IgG) through non-covalent interactions to form a very stable complex. Using fluorescent substances to label ferritin, the specific binding process of the protein complex to cancer cells can be observed.
Viral Nanocarriers
Many viruses derived from microorganisms, animals, and insects can be used to prepare nanocarriers for drug delivery. Drugs are loaded on the surface or inside of viral nanocarriers (VLPs) and can be effectively internalized by cells. For example, a filamentous bacteriophage (M13) composed of five protein coats is an extensively studied nanocarrier.
Cell Penetrating Peptide
Cell penetrating peptide (CPP) is a class of short peptides extracted from microorganisms, phages, etc., which have the function of penetrating biomembranes and can mediate transmembrane transduction of biomacromolecules. In 1988, researchers first discovered TAT peptide from human immunodeficiency virus (HIV), that is, human immunodeficiency virus type I transcription activator, which can enter cells efficiently and has little cytotoxicity. Since then, scientists have been interested in using CPPs for intracellular drug delivery.
Exosomes
Exosomes are lipoprotein nanovesicles secreted by cells that can interact with cell membranes. Functional proteins and ligands attached to the surface of exosomes can effectively deliver substances loaded with drug molecules to target cells, so exosomes are considered to be a promising drug carrier. As we all know, catalase is a substance specifically expressed in Parkinson’s patients. Studies have shown that exosomes secreted by monocytes and macrophages can avoid the phagocytosis of mononuclear phagocytes. Based on the above theory, the researchers tried to embed catalase in exosomes as a nano-drug carrier for the treatment of Parkinson’s disease. This system can improve the delivery efficiency of drugs to target cells, thereby improving the therapeutic effect of drugs.
Nanoparticles Made of Natural Polymers
Polymer-based nanocarriers can be divided into natural polymers and synthetic polymers. Polysaccharides such as CS, sodium alginate, glycosides and their derivatives are an important class of natural polymer materials, which are widely used in the delivery of intracellular drug molecules due to their good biocompatibility and biodegradability. CS is a class of natural polymers isolated from the excrement of crustaceans and fungi. It has good biocompatibility, degradability, low toxicity, antibacterial and adhesion properties, and is widely used in the field of biochemistry. The CS-based polymer electrolyte complex is prepared by the interaction between positively charged CS and negatively charged chlorinated polyethylene (PEC). Since it does not require organic solvents or sonication, it is considered a mild drug delivery method.
More interestingly, there are free protonatable amino groups on the glucosamine unit of CS, resulting in different solubility at different pH values, which largely affects the delivery of drug molecules. Through modification methods such as alkylation, thiolation, carboxylation, and quaternization, different CS derivatives can be designed to skillfully solve the problem of poor water solubility of natural CS at different pH values, and at the same time improve its stability and Adhesive. In addition, CS can also be chemically modified with some sulfhydryl reagents such as glutathione, cysteine, thioglycolic acid, etc. to synthesize sulfhydryl chitosan. The thiol functional group can bind to the cysteine-rich glycoprotein of the mucus layer, thereby effectively promoting the adhesion of the intestinal wall mucus. Chitosan derivatives specially modified with amino acids have different anticoagulant abilities and cell penetration functions under neutral, acidic, and alkaline conditions, which is of great significance for solving drug delivery.