Latent TB Testing Market Size, Share, Trends & Forecast to (2024-2032) | UnivDatos
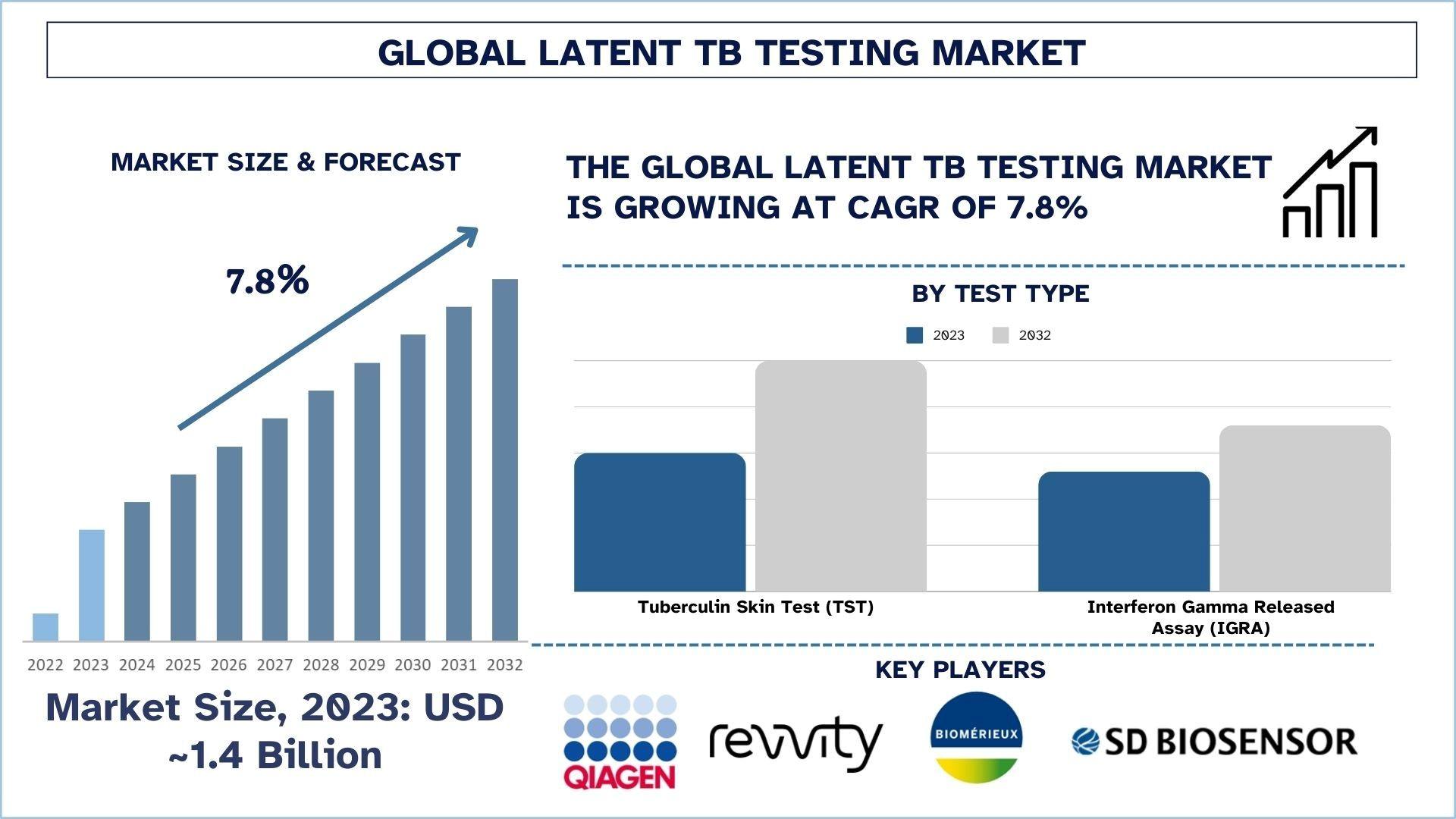
According to the UnivDatos, rising tuberculosis (TB) incidence, increased need for screening in HIV/AIDS and transplant patients, rising advanced diagnostics, and adoption of IGRAs drive the Latent TB Testing market. As per their “Latent TB Testing Market” report, the global market was valued at USD 1.4 Billion in 2023, growing at a CAGR of about 7.8% during the forecast period from 2024 - 2032 to reach USD Billion by 2032.
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/latent-tb-testing-market?popup=report-enquiry
Tuberculosis (TB) is still one of the world’s deadliest infectious diseases that kills millions of people every year. A lot has been achieved in the diagnosis and management of active TB, however, the LTBI remains a concealed danger. Screening tests to detect LTBI are now an indispensable tool in the TB elimination strategy because such individuals are Mycobacterium tuberculosis carriers with no disease manifestation. The recent developments and changes in policies this year have created focus and entrance to this market making it a player in the fight against TB.
A total of 1.25 million people died from tuberculosis (TB) in 2023 (including 161 000 people with HIV). Worldwide, TB has probably returned to being the world’s leading cause of death from a single infectious agent, following three years in which it was replaced by coronavirus disease (COVID-19). It was also the leading killer of people with HIV and a major cause of deaths related to antimicrobial resistance.
In 2023, an estimated 10.8 million people fell ill with TB worldwide, including 6.0 million men, 3.6 million women and 1.3 million children. TB is present in all countries and age groups. TB is curable and preventable.
Multidrug-resistant TB (MDR-TB) remains a public health crisis and a health security threat. Only about 2 in 5 people with drug resistant TB accessed treatment in 2023.
According to NIH estimates, almost 33% of people worldwide have latent tuberculosis infections (LTBI) in 2023.
Global TB Burden and the Role of Latent TB Testing
These are people who may develop active TB disease as they are part of those considered to be at high risk for the disease, including PLHIV, health care workers, first-degree contacts of persons with TB, or those who were previously infected with TB. The need for latent TB testing has increased in essence, as agencies reach for better control and eventual elimination of TB, by seeking cases and eradicating them before their potential to spread is realized.
Developments in Testing as a Technology
On November 25, 2023 Revvity, a global company that leverages innovation in Diagnostics and Life Sciences announced the country launch of its T-SPOT.TB test for latent TB screening, at MICROCON in Lucknow.
New developments in the classifications of technological breakthroughs therefore have greatly enhanced the testing for latent TB. In contrast to the TST which is sensitive to previous BCG vaccination, IGRAs rarely give a false-positive result. Industry including QuantiFERON-TB Gold Plus and T-SPOT.TB has established main products in the developed countries wherein high accuracy and reliability are valued.
In March 2022, PerkinElmer, Inc., (NYSE: PKI) a global leader committed to innovating for a healthier world, announced that the Company has completed its previously announced acquisition of Oxford Immunotec Global PLC (Oxford Immunotec). PerkinElmer originally announced its intent to acquire Oxford Immunotec on January 7, 2021.
Diagnostic companies are also discussing new opportunities for development – point of care solutions for IGRA and integration into other digital health applications. These developments are intended to extend possibilities of LTBI diagnosis to better diagnose populations in environments where resources are scarce, and tuberculosis is most abundant.
Government Measures to Foster Growth
On March 23, 2023, PerkinElmer’s Oxford Immunotec announced that the U.S. Food and Drug Administration (FDA) has approved the use of two additional cell isolation instruments with the Company’s previously approved T-Cell Select™ reagent kit, which is intended for in vitro diagnostic (IVD) use by certified laboratories with the T-SPOT®.TB test workflow.
This market is growing due to government policies and international health-related organizations stimulating the latent TB testing market. For example, CDC/WHO urges primary latent TB screening for an increased-risk population. Similarly, TB elimination programs in Europe and North America have incorporated latent TB testing into immigration and occupational health services.
As demonstrated in Asia and Africa's high-burden areas, collaborations between governments, non-governmental organizations, and private organizations of the private sector are increasingly focusing on increasing access to testing. They include subsidized, mobile testing units and communal outreach activities. They include the emerging awareness of the validity of using properly sputum-indicated TST for TB control due to the program’s cheap price.
Way Forward
The future of latent TB testing is constantly evolving and more efficient and accessible as well as critical advances and multi-disciplinary cooperation. Higher diagnostic equipment, along with strong public health measures, are expected to enter the market in the future. Both affordability and awareness will, therefore, need stakeholder efforts from all domains of the healthcare value chain technologies.
As World efforts enhance towards the fight against TB, then there will be continued demand for latent TB testing, as part of population health strategies. With the help of diagnosing and curing the TB germs the market is working on making the world free of Tuberculosis taking millions breathing and freeing the economy from the burden of such diseases.
Click here to view the Report Description & TOC :https://univdatos.com/reports/latent-tb-testing-market
Conclusion
Screening for latent TB takes a central place in international initiatives aimed at controlling TB primarily because many individuals suffering from the infection develop active conditions shortly after. Various diagnostic technologies are emerging as governments’ support and strategic collaborations encourage applications that make diagnostics more accessible and accurate. However, problems like financial constraints and low sensitization in high-impact areas need to be met. Immersed in constant innovation and partnership, latent TB testing can look forward to having a huge impact on the global spread of TB and helping to save millions of people.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - [email protected]
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



