AI/ML-Enabled Medical Devices Market Size, Share, Trends & Forecast to (2024-2032) | UnivDatos
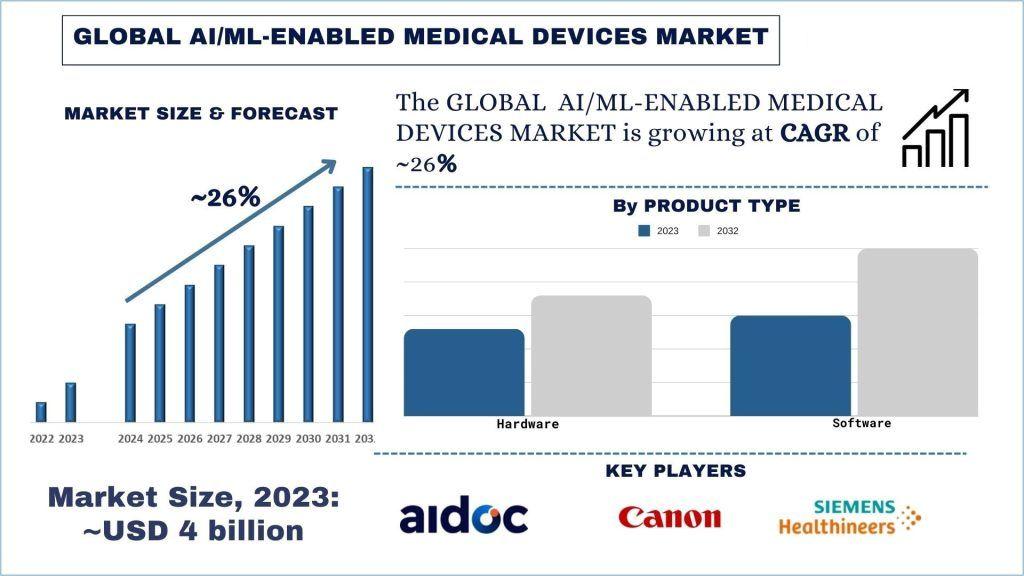
According to the UnivDatos, the surge in product launches and the demand for smart medical devices will drive the global scenario of the AI/ML-enabled medical devices market. As per their “AI/ML-Enabled Medical Devices Market” report, the global market was valued at ~USD 4 billion in 2023, growing at a CAGR of about 26% during the forecast period from 2024 - 2032 to reach USD billion by 2032.
The North American market for artificial intelligence/ machine learning-enabled medical devices is poised for continued growth, driven by ongoing technological innovation, a favorable regulatory environment, increasing demand for personalized healthcare, and the expanding adoption of digital health solutions across the healthcare ecosystem. Here's a detailed overview:
Access sample report (including graphs, charts, and figures): https://univdatos.com/reports/ai-ml-enabled-medical-devices-market?popup=report-enquiry
Technological Advancements: North America, particularly the U.S., is home to leading technology companies, research institutions, and healthcare organizations driving innovation in AI and ML-enabled medical devices. Significant advancements in AI algorithms, computing power, and data analytics have enabled the development of sophisticated medical devices capable of analyzing complex datasets and providing actionable insights to healthcare providers.
Increasing Adoption of Digital Health Solutions: There is a growing recognition of the potential benefits of digital health solutions, including AI and ML-enabled medical devices, in improving healthcare delivery, enhancing patient outcomes, and reducing costs. Healthcare providers in North America are increasingly embracing these technologies to streamline workflows, improve diagnostics, and deliver more personalized care to patients.
Supportive Regulatory Environment: Regulatory agencies such as the Food and Drug Administration (FDA) in the U.S. have established frameworks to facilitate the development and approval of AI and ML-enabled medical devices. Initiatives such as the FDA's Digital Health Innovation Action Plan and Pre-Certification Program provide pathways for expedited review and approval of innovative digital health technologies, encouraging investment and innovation in the sector.
Growing Demand for Personalized Healthcare: Patients in North America are increasingly seeking personalized healthcare solutions that consider their unique medical history, genetic makeup, and lifestyle factors. AI and ML-enabled medical devices enable healthcare providers to deliver more targeted and effective treatments tailored to individual patient needs, driving demand for these technologies in the region.
Strategic Partnerships and Collaborations: Collaboration between technology companies, healthcare providers, and research institutions has accelerated the development and adoption of AI and ML-enabled medical devices in North America. Strategic partnerships enable companies to leverage complementary expertise, resources, and networks to bring innovative products to market and expand their reach within the healthcare industry.
Investment and Funding: North America attracts significant investment and funding for AI and ML-enabled medical device startups and companies. Venture capital firms, private equity investors, and government agencies provide capital to support research, development, and commercialization efforts, fueling growth and innovation in the market.
In March 2024, Siemens Medical Solutions USA, Inc. received FDA authorization for its device NAEOTOM Alpha in the radiology sector.
In October 2023, U.S-based GE HealthCare stood first in the FDA’s AI-enabled medical device authorization list.
In March 2024, U.S-based Beacon Biosignals, Inc. received FDA authorization for its device SleepStageML in the neurology sector.
Upright and Steady Climb: The AI/ML-enabled medical devices market in North America thrives due to the above-mentioned factors. These factors collectively contribute to the region's enduring pair-up with the AI/ML-enabled medical devices and its sustained growth in popularity. AI/ML-enabled medical devices have already made their mark in the healthcare market. As this dynamic market continues to develop and grow, it provides hope for the global effort to create innovative medical devices. The AI/ML-enabled medical devices domain is constantly innovating and redefining its innovative system from the ground up.
Click here to view the Report Description & TOC : https://univdatos.com/reports/ai-ml-enabled-medical-devices-market
Conclusion:
The AI/ML-enabled medical devices market is still in its early stages due to the rapid development and expansion of the medical device industry. This indicates the ongoing efforts to modify the product portfolio of medical devices globally, which are gradually changing the landscape. Furthermore, the increased product launches in the sector further expand the market's potential. Despite its unique challenges, the world is progressing toward developing more innovative and user-friendly AI/ML-enabled medical devices. As this nascent market continues to grow and develop, it has the potential to contribute significantly to global efforts to combat many of the restraints associated with it. Despite the hurdles, the future of AI/ML-enabled medical devices is undeniably bright. A new dawn is breaking in the development of innovative devices. There's no denying that these instruments are transforming the healthcare industry’s outlook, bringing enormous varieties to the population worldwide.
Contact Us:
UnivDatos
Contact Number - +1 978 733 0253
Email - [email protected]
Website - www.univdatos.com
Linkedin- https://www.linkedin.com/company/univ-datos-market-insight/mycompany/
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Giochi
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Altre informazioni
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness



